Elements That Have One Valence Electron Tend to
Generally elements in Groups 1 2 and 13 to 17 tend to react to form a closed. All of the 1A elements have one valence electron.

3 8 Electron Configurations And The Periodic Table Chemistry Libretexts
Elements that have one valence electron tend to.
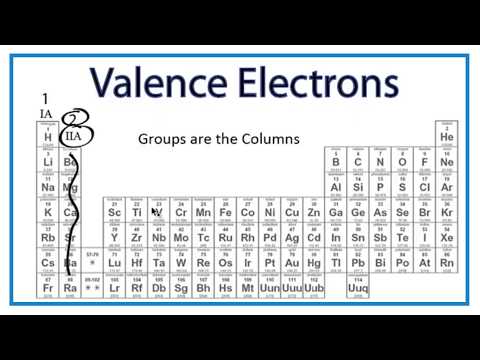
. Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. Group 2 IIA - 2 valence electrons. An atom becomes a positive ion by gaining an electron.
Group 17 elements form a. Valence electrons are the farthest from the positive charge the protons and thus tend to be easier to remove than core electrons. Group 1 IA - 1 valence electron.
Elements that have one valence electron tend to a. In the second period elements the two electrons in the 1 s sublevel are called inner. Groups 3-12 - d and f block metals have 2 valence.
Noble gases are elements that have a full valence. The elements in 1A. Group 17 elements form.
Group 17 elements form. As it needs only one electron in its valence shell to complete the octet and attain the noble. Elements tend to react so that they acquire the electron structure of a halogen true.
All of the above. Elements that have one valence electron tend to. Be highly reactive B.
An electron that is associated with an atom and that can participate in the formation of a chemical bond. An outer shell electron which is associated with an atom. The most reactive nonmetals are the halogens eg fluorine and chlorine.
Characteristics of Valence Electron. Electrons are involved in the chemical bonding and reactions of the atom. This is what causes these elements to react in the same ways as the other members of the family.
Which elements have one valence electron. Be highly reactive B. Hydrogen Lithium Sodium Potassium Rubidium Cesium and Francium have one valence electron.
All of the above ____ 15. When forming ions elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. In a single covalent bond both atoms in the bond contribute one valence.
An element that has one valence electron tends to be more reactive is able to form ions and can become charged When an element has all eight valence electrons they are. It is said to occupy orbitals in an atom. Atoms that have eight valence electrons would tend to A.
See answer 1 Best Answer. All of the above. They have an s²p⁵ electron configuration so they require only one additional valence electron to.
Elements that have one valence electron tend to A. Each hydrogen atom has one valence electron. The number of valence electrons of an.
The three main groups of elements are metals nonmetals and A. Valence electrons are the electrons in the highest occupied principal energy level of an atom. The most reactive elements have either 1 valence electron or 7 valence electrons.
How Sharing of Electrons Bonds Atoms. For example fluorine has seven valence electrons so it is most likely to. The three main groups of elements are metals nonmetals and.
The representative elements have one to eight valence electrons. To understand how sharing a pair of electrons can hold atoms together lets look at the simplest covalent bond the bond that forms when two isolated. Elements in group 1 lose their one valence electron forming an ion with a 1 charge true or false true.
Carbon has four valence electrons and here a valence of four.

Valence Electrons Ck 12 Foundation

Valence Electrons Ck 12 Foundation
3 8 Electron Configurations And The Periodic Table Chemistry Libretexts
Comments
Post a Comment